Unveiling The Mysteries Of 16 Sodium Atoms: What You Need To Know
Alright, buckle up, folks! We’re diving deep into the world of chemistry, and today, we’re talking about something that might sound simple but holds some fascinating secrets—16 sodium atoms. If you’ve ever wondered what sodium atoms are, why they matter, and how 16 of them could change the game, you’re in the right place. So, grab your lab goggles, and let’s get started, shall we?
Now, you might be thinking, "Why 16 sodium atoms? Isn’t that just some random number?" Well, my friend, it’s not as random as you think. Sodium is one of the most essential elements on the periodic table, and when you start grouping these atoms together, especially in sets of 16, things get interesting. From biological functions to industrial applications, sodium plays a crucial role in our daily lives.
In this article, we’ll break down everything you need to know about 16 sodium atoms. We’ll explore their properties, significance, and even touch on some mind-blowing facts that will leave you saying, "Whoa, I didn’t know that!" So, whether you’re a science enthusiast or just someone curious about the world around you, this article has got you covered.
- Das Geheimnis Um Sofia Levander Alles Ber Daniel Eks Frau
- Webserien Download Die Besten Plattformen Tipps Fr Dich
What Are Sodium Atoms, Anyway?
Before we jump into the specifics of 16 sodium atoms, let’s take a step back and understand what sodium atoms actually are. Sodium, represented by the symbol Na on the periodic table, is a soft, silvery-white metal that belongs to the alkali metal group. It’s highly reactive and plays a vital role in various biological processes. But what makes sodium atoms so special?
Each sodium atom consists of 11 protons, 11 electrons, and 12 neutrons. This unique composition gives sodium its distinct properties, such as its ability to conduct electricity and its reactivity with water. And when you group 16 of these atoms together, the possibilities become endless.
Why 16 Sodium Atoms Matter
Now, here’s where things get exciting. When you have 16 sodium atoms, you’re not just talking about a random cluster of elements. These atoms can form structures that have specific applications in science and technology. For instance, they can be used in advanced materials, energy storage, and even medical imaging.
- Der Zwilling Mann Alles Ber Seine Eigenschaften Geheimnisse
- Alyx Star Ihr Aufstieg Ihre Karriere Ihr Vermgen Alles Ber Sie
- Advanced materials: Sodium clusters can enhance the properties of materials used in electronics and aerospace.
- Energy storage: Sodium-ion batteries are gaining popularity as a cost-effective alternative to lithium-ion batteries.
- Medical imaging: Sodium atoms can be used in MRI scans to detect certain conditions in the human body.
So, as you can see, 16 sodium atoms are more than just a number—they’re a gateway to innovation and discovery.
Properties of 16 Sodium Atoms
Let’s dive deeper into the properties of 16 sodium atoms. When sodium atoms cluster together, they exhibit unique characteristics that differ from individual atoms. These clusters can form stable structures with specific electronic and magnetic properties.
One of the most fascinating aspects of sodium clusters is their size-dependent properties. As the number of atoms in a cluster increases, the properties of the cluster change. For example, a cluster of 16 sodium atoms might have different electronic configurations compared to a cluster of 10 or 20 atoms. This makes them highly versatile for various applications.
Electronic Properties
The electronic properties of 16 sodium atoms are particularly intriguing. These clusters can exhibit metallic behavior, making them excellent conductors of electricity. They can also form stable electron configurations that make them ideal for use in nanotechnology.
Moreover, the electronic structure of sodium clusters can be manipulated by changing the number of atoms or introducing other elements. This opens up a world of possibilities for creating customized materials with specific properties.
Applications of 16 Sodium Atoms
Now that we’ve explored the properties of 16 sodium atoms, let’s talk about their applications. From energy storage to medical imaging, these clusters are making waves in various industries. Here are some of the most exciting applications:
Energy Storage
Sodium-ion batteries are becoming increasingly popular due to their cost-effectiveness and abundance of raw materials. Unlike lithium, which is relatively scarce, sodium is abundant and affordable. By using clusters of 16 sodium atoms, scientists can optimize the performance of these batteries, making them more efficient and reliable.
Medical Imaging
In the field of medical imaging, sodium atoms are used in MRI scans to detect sodium ions in the body. This can help diagnose conditions such as brain tumors and strokes. By using clusters of 16 sodium atoms, researchers can improve the sensitivity and accuracy of these scans, leading to better diagnoses and treatment plans.
The Science Behind Sodium Clusters
So, how do these clusters of 16 sodium atoms form, and what makes them so special? The science behind sodium clusters is a fascinating blend of chemistry and physics. When sodium atoms come together, they form stable structures through a process known as self-assembly. This process is driven by the interactions between the atoms and their environment.
One of the key factors that influence the formation of sodium clusters is the size of the cluster. As the number of atoms in a cluster increases, the properties of the cluster change. For example, a cluster of 16 sodium atoms might have different electronic and magnetic properties compared to a cluster of 10 or 20 atoms. This size-dependent behavior makes sodium clusters highly versatile for various applications.
Quantum Effects
At the nanoscale, quantum effects become significant, and sodium clusters are no exception. These effects can influence the electronic and magnetic properties of the clusters, making them behave differently from bulk materials. By understanding these quantum effects, scientists can design materials with specific properties for various applications.
Challenges and Opportunities
While the potential applications of 16 sodium atoms are exciting, there are also challenges to overcome. One of the main challenges is controlling the size and shape of the clusters. Achieving uniformity in size and shape is crucial for optimizing the properties of the clusters for specific applications.
Another challenge is stability. Sodium clusters can be highly reactive, especially when exposed to air or moisture. This can limit their use in certain environments. However, researchers are working on ways to stabilize these clusters, such as coating them with protective layers or using them in controlled environments.
Opportunities for Innovation
Despite these challenges, the opportunities for innovation are vast. By overcoming these hurdles, scientists can unlock the full potential of 16 sodium atoms. From developing more efficient energy storage solutions to improving medical imaging techniques, the possibilities are endless.
Real-World Examples
To give you a better idea of how 16 sodium atoms are being used in the real world, let’s look at some examples:
- Sodium-ion batteries: Companies like Faradion and Tiamat are developing sodium-ion batteries that use clusters of sodium atoms to improve energy storage efficiency.
- MRI imaging: Researchers at the University of California, San Diego, are using sodium clusters to enhance the sensitivity of MRI scans for detecting brain tumors.
- Nanotechnology: Scientists at the University of Cambridge are exploring the use of sodium clusters in nanomaterials for various applications, from electronics to aerospace.
These examples demonstrate the real-world impact of 16 sodium atoms and highlight the importance of continued research in this field.
Future Directions
As we look to the future, the possibilities for 16 sodium atoms are endless. With advancements in nanotechnology and materials science, we can expect to see even more innovative applications. From developing smarter energy storage solutions to creating more accurate medical imaging techniques, the potential is truly remarkable.
Moreover, as researchers gain a deeper understanding of the properties of sodium clusters, they can design materials with specific properties for various applications. This could lead to breakthroughs in fields such as electronics, aerospace, and healthcare.
Conclusion
And there you have it, folks! We’ve explored the fascinating world of 16 sodium atoms, from their properties and applications to the challenges and opportunities they present. Whether you’re a scientist, engineer, or just someone curious about the world around you, 16 sodium atoms offer a glimpse into the future of innovation and discovery.
So, what’s next? Well, we’d love to hear your thoughts! Leave a comment below and let us know what you think about the potential applications of 16 sodium atoms. And if you enjoyed this article, don’t forget to share it with your friends and family. Who knows? You might just inspire the next big breakthrough!
Table of Contents
- What Are Sodium Atoms, Anyway?
- Why 16 Sodium Atoms Matter
- Properties of 16 Sodium Atoms
- Applications of 16 Sodium Atoms
- The Science Behind Sodium Clusters
- Challenges and Opportunities
- Real-World Examples
- Future Directions
- Conclusion
- Isabel May Ist Sie Single 2023 Alles Ber Ihre Karriere Amp Privatleben
- Filmywap Co Ist Kostenlos Wirklich Gratis Gefahren Alternativen
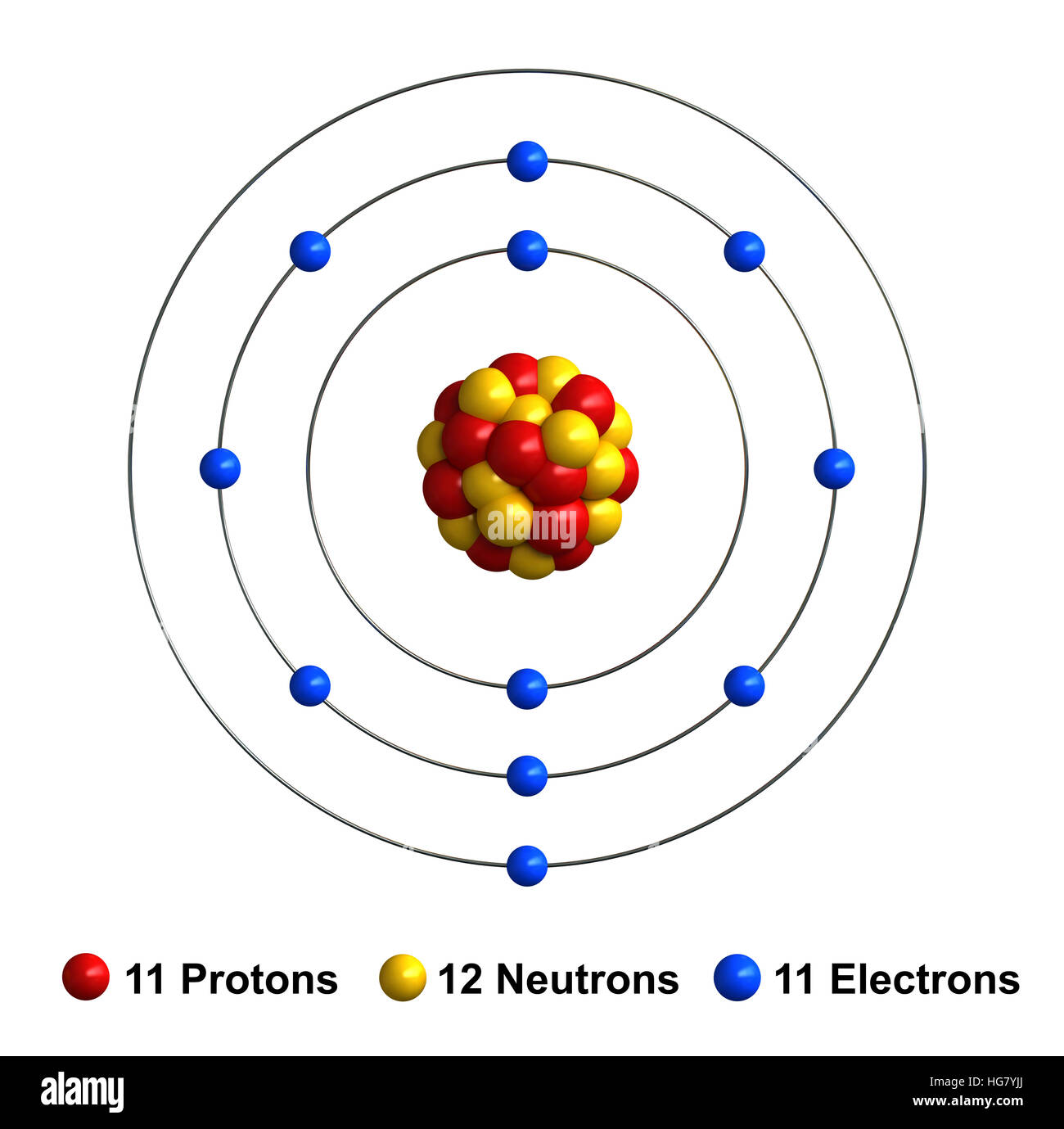
3d render of atom structure of sodium isolated over white background
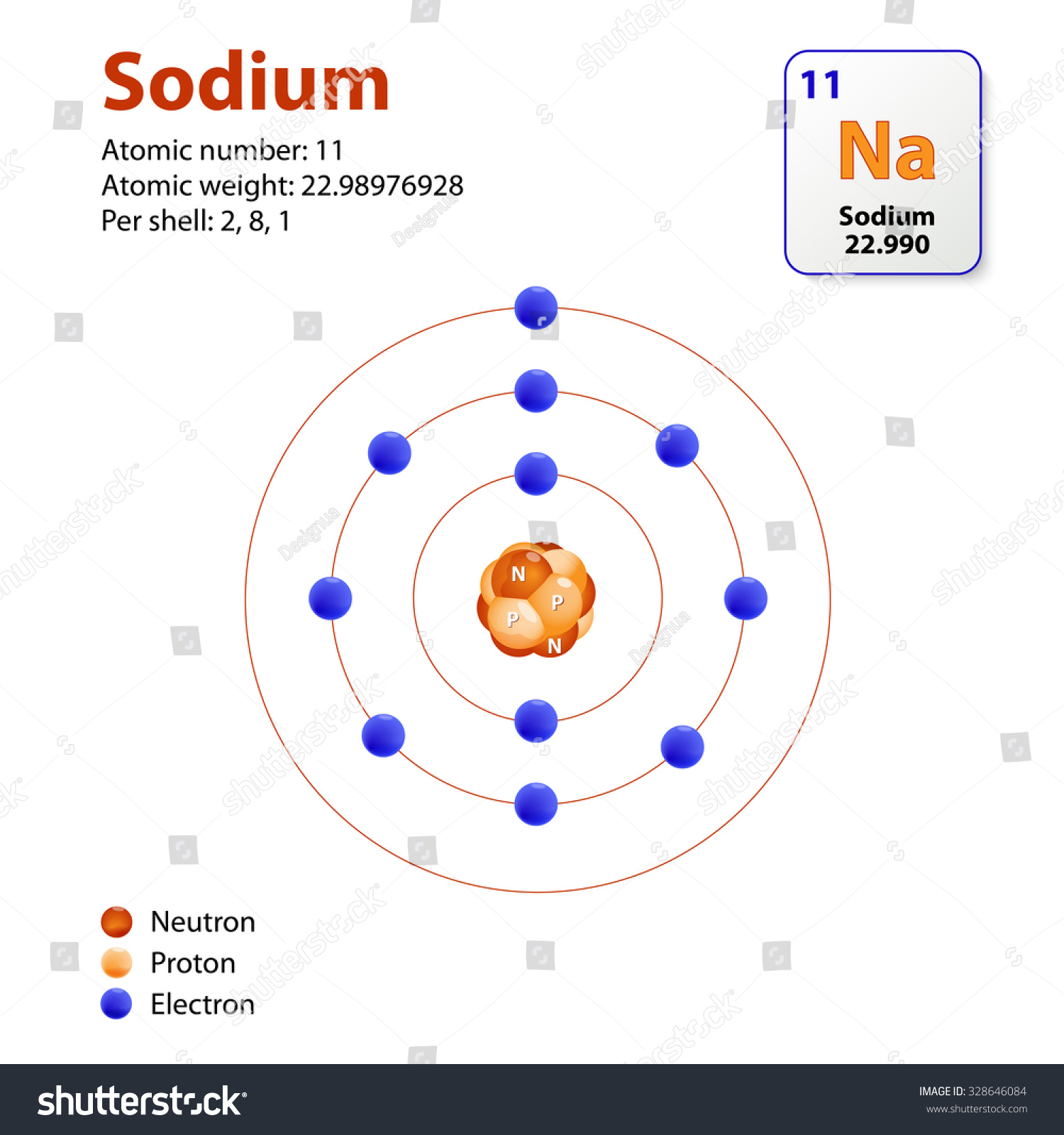
This Diagram Shows The Electron Shell Configuration For The Sodium Atom

3d Bohr Model Of Sodium